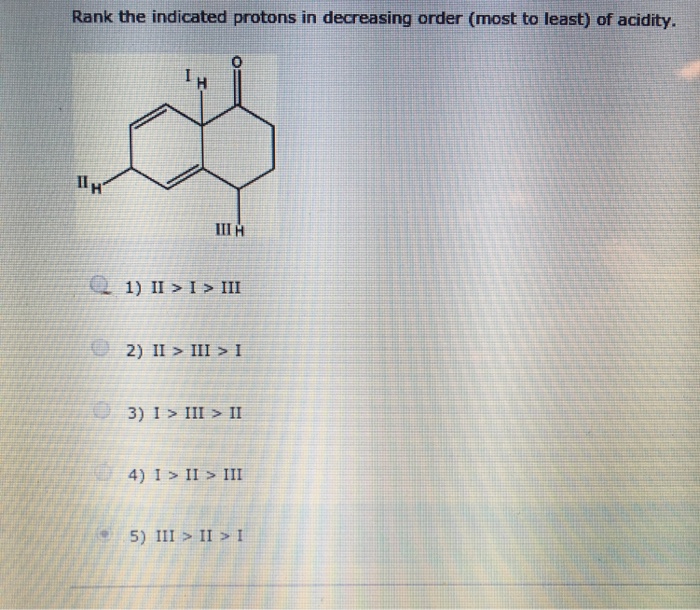
Rank the indicated protons in decreasing order of acidity to unravel the intricacies of proton acidity and stability. This concept is fundamental to understanding chemical reactions and the behavior of acids and bases.
Factors such as resonance, inductive effects, hybridization, orbital overlap, and solvent effects play crucial roles in determining proton acidity. By exploring these factors, we gain insights into the stability of conjugate bases and the reactivity of acids.
Proton Acidity and Stability
Proton acidity refers to the ability of a compound to donate a proton (H+). The stronger the acid, the more readily it donates a proton. Proton acidity is influenced by several factors, including the stability of the conjugate base, resonance, inductive effects, hybridization, and orbital overlap.
Strong acids have conjugate bases that are very stable, meaning they are not likely to accept a proton back. Weak acids have conjugate bases that are less stable and are more likely to accept a proton back.
Factors Affecting Proton Acidity
- Stability of the conjugate base:The more stable the conjugate base, the stronger the acid.
- Resonance:Resonance can stabilize the conjugate base, making the acid stronger.
- Inductive effects:Inductive effects can either increase or decrease the acidity of a proton.
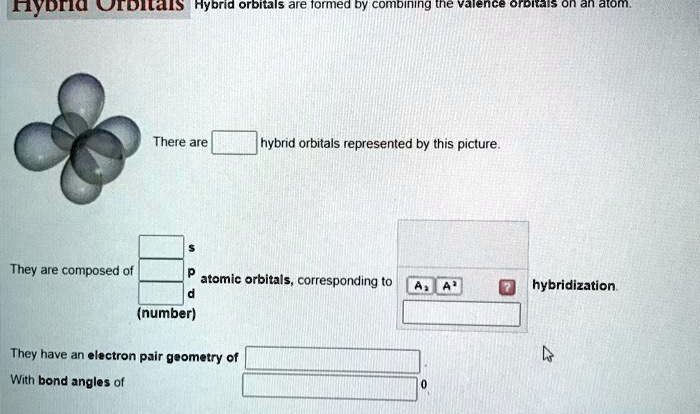
- Hybridization:The hybridization of the atom bonded to the proton can affect its acidity.
- Orbital overlap:The extent of orbital overlap between the proton and the atom bonded to it can affect its acidity.
Resonance and Delocalization
Resonance is a phenomenon that occurs when there are multiple possible Lewis structures for a molecule. In such cases, the actual structure of the molecule is a hybrid of the resonance structures.
Resonance can stabilize the conjugate base of an acid by delocalizing the negative charge over multiple atoms. This makes the conjugate base less likely to accept a proton back, which in turn makes the acid stronger.
Examples of Resonance-Stabilized Conjugate Bases, Rank the indicated protons in decreasing order of acidity
- The conjugate base of acetic acid (CH3COOH) is the acetate ion (CH3COO-). The acetate ion is resonance-stabilized, which makes acetic acid a relatively strong acid.
- The conjugate base of phenol (C6H5OH) is the phenolate ion (C6H5O-). The phenolate ion is also resonance-stabilized, which makes phenol a relatively strong acid.
Inductive Effects
Inductive effects are the result of the polarization of electrons in a molecule. When an electronegative atom is bonded to a less electronegative atom, the electrons in the bond are pulled towards the electronegative atom. This creates a partial positive charge on the less electronegative atom and a partial negative charge on the electronegative atom.
Inductive effects can either increase or decrease the acidity of a proton. If the electronegative atom is bonded to the proton, the inductive effect will decrease the acidity of the proton. This is because the electronegative atom will pull electrons away from the proton, making it less likely to be donated.
Examples of Inductive Effects on Proton Acidity
- The inductive effect of the chlorine atom in HCl increases the acidity of the proton. This is because the chlorine atom is more electronegative than hydrogen, which pulls electrons away from the proton.
- The inductive effect of the methyl group in CH3CH2OH decreases the acidity of the proton. This is because the methyl group is less electronegative than hydrogen, which pushes electrons towards the proton.
Hybridization and Orbital Overlap: Rank The Indicated Protons In Decreasing Order Of Acidity
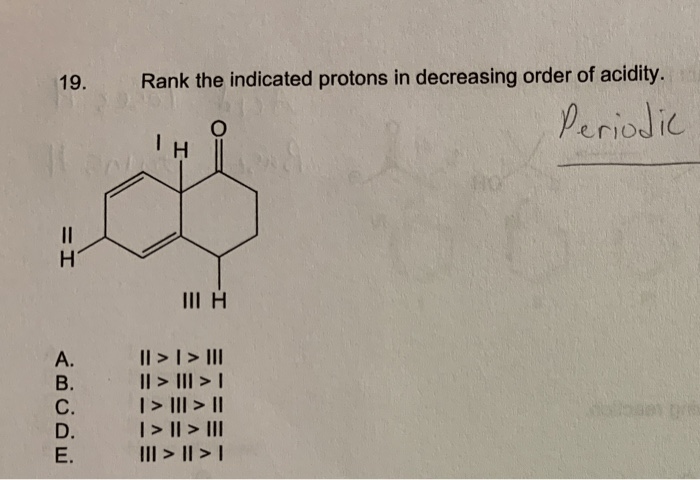
The hybridization of the atom bonded to the proton can affect its acidity. The more s-character in the hybridization, the more acidic the proton will be.
This is because s-orbitals are more electronegative than p-orbitals. When an atom is hybridized with more s-character, the electrons in the bond are pulled closer to the atom, which makes the proton more likely to be donated.
Examples of Hybridization and Orbital Overlap Effects on Proton Acidity
- The proton on a carbon atom that is sp3-hybridized is less acidic than the proton on a carbon atom that is sp2-hybridized. This is because the sp3-hybridized carbon atom has more s-character, which pulls electrons closer to the carbon atom and makes the proton less likely to be donated.
- The proton on a carbon atom that is sp2-hybridized is less acidic than the proton on a carbon atom that is sp-hybridized. This is because the sp2-hybridized carbon atom has more s-character, which pulls electrons closer to the carbon atom and makes the proton less likely to be donated.
Solvent Effects
The solvent in which an acid is dissolved can also affect its acidity. Solvents that are able to donate electrons can stabilize the conjugate base of an acid, which in turn makes the acid stronger.
Solvents that are able to accept electrons can destabilize the conjugate base of an acid, which in turn makes the acid weaker.
Examples of Solvent Effects on Proton Acidity
- Acetic acid is a stronger acid in water than it is in benzene. This is because water is able to donate electrons to the acetate ion, which stabilizes it and makes acetic acid a stronger acid.
- Phenol is a weaker acid in water than it is in benzene. This is because water is able to accept electrons from the phenolate ion, which destabilizes it and makes phenol a weaker acid.
Essential Questionnaire
What is proton acidity?
Proton acidity refers to the tendency of a proton (H+) to be released from a molecule or ion.
How does resonance affect proton acidity?
Resonance stabilizes conjugate bases by delocalizing the negative charge, making the proton more acidic.
What is the relationship between hybridization and proton acidity?
Hybridization affects the orbital overlap between the proton and the neighboring atoms, influencing the proton’s acidity.